Scientists say too much carbon dioxide is bad for the Earth. And too much carbon monoxide can kill you. So why are researchers at the University of Michigan excited about turning CO2 into CO? Because the end product could come in handy for producing electricity and hydrogen. U of M chemists, along with others from the University of Oxford, say they've come up with an efficient way to turn carbon dioxide into carbon monoxide using visible light (like sunlight).
Apparently, they're not the first ones to cite the opportunities this creates. Researchers at Sandia National Laboratories in New Mexico boasted in late 2007 about using concentrated solar energy to convert CO2 back to CO.
What's different here? The U of M/Oxford method, reported in the Journal of the American Chemical Society, apparently uses considerably less energy input than current methods. And, it's pretty close to what nature does naturally.
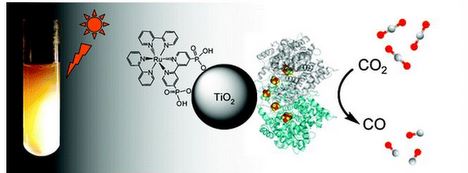
Credit: JACS.
"This is a first step in showing it's possible, and imagine microbes doing something similar," says U of M biological chemist Steve Ragsdale.
"I don't know of any organism that uses light energy to activate carbon dioxide and reduce it to carbon monoxide, but I can imagine either finding an organism that can do it, or genetically engineering one to channel light energy to coax it to do that."
Ragsdale and others used an enzyme-modified titanium oxide and a photosensitizer to make the conversion to carbon monoxide. They say the direct product can be used not only to produce electricity or hydrogen but also can be converted by known catalysts into liquid fuel.
Two things to note: The conversion needs to be done in an oxygen-free environment, and the carbon monoxide that's created needs to be managed (because of the killing people part).
Ragsdale also is a fellow of the Michigan Memorial Phoenix Energy Institute, which develops, coordinates and promotes energy research and education at U of M.
The research was funded by the National Institute of General Medical Sciences.
Is this an answer to the so-called climate crisis of human-induced global warming from the burning of fossil fuels?
According to F.A. Armstrong, from the Oxford Department of Chemistry: "This is a reaction of great technological importance. Otherwise (at synthetic catalysts) the process is so inefficient and slow that it has made little impact on industry."
More from TreeHugger
Fuel Made by Sun, Water, Carbon Dioxide, and Nanotechnology
Where Does all the Carbon Dioxide End Up?
The Silly Season Is Upon Us: Fiorina Goes After Boxer on Climate Action


